Difference between revisions of "Guide to Atmospherics"
imported>Kingofkosmos |
imported>Kingofkosmos |
||
| Line 135: | Line 135: | ||
===Optimizing Internals=== | ===Optimizing Internals=== | ||
* On a basic view, a 16 kPa minimum O2 requirement in internals. Pure O2 is theoretically toxic in real life, but has no representation for this in code, and takes a while to be really dangerous anyway (they use it to treat certain diseases, for example), and thus using an air tank for internals is fairly inefficient. | * On a basic view, a 16 kPa minimum O2 requirement in internals. Pure O2 is theoretically toxic in real life, but has no representation for this in code, and takes a while to be really dangerous anyway (they use it to treat certain diseases, for example), and thus using an air tank for internals is fairly inefficient. | ||
| − | * Cold air has more moles per kPa, and because people breath in moles, and filling tanks usefully for internals is largely capped by the 1000 kPa release pressure, which means cooling your air before using in internals is important. Cooled down air, such as from a freezer-ed canister, is the most efficient way to set up internals. Cooling it below | + | * Cold air has more moles per kPa, and because people breath in moles, and filling tanks usefully for internals is largely capped by the 1000 kPa release pressure, which means cooling your air before using in internals is important. Cooled down air, such as from a freezer-ed canister, is the most efficient way to set up internals. Cooling it below 264 K will result in icicles inside lungs! |
* If you need to empty an internal tank to make space for better, colder air, you can use an air pump. Set it to 'pump in' and 'turn on' then 'off' with the tank inside it, allowing you to refill the tank more effectively. | * If you need to empty an internal tank to make space for better, colder air, you can use an air pump. Set it to 'pump in' and 'turn on' then 'off' with the tank inside it, allowing you to refill the tank more effectively. | ||
| − | |||
==Fun Projects== | ==Fun Projects== | ||
Revision as of 00:46, 12 November 2013
This is the Advanced Atmospherics Guide. If you're looking for a Quick Setup Guide, see this. If you're ready to really learn about the atmospheric system, read on. By reading this guide you will learn how to transform Atmos from a waste of space to an actually useful addition. We will go through all kinds of theory, so this may be tough, but it will also ensure you know exactly how and more importantly why Atmos works the way it does, making you ready for all kinds of situations.
Other Atmospherics related information:
Let's start with some theory about the gases.
The Gases and Their Functions
These are the different gases that can be found in game.
Air
A 1/5 gasmix of O2 and N2 (20% O2, 80% N2). The station is filled with this. Air in SS13 can be seen, strangely enough, as a 'watered down'-O2, with N2 being the water. Optimal pressure for humans is 101.3 kPa. Due to the minimum of 16 kPa of O2, the pressure of 101.3 kPa cannot be changed too much without the situation becoming excessively lethal. Under 16% oxygen? You start dying. Under 90 kPa due to fire from a while ago? You start dying. Be mindful of this.
O2
Oxygen. You breathe this. Running out of O2 will cause your slow death by suffocation damage. It is also required for a fire to even start, and hold, ending the fire when the O2 or Plasma is depleted. Having less than 16 kPa of O2 flowing into your lungs chokes you.
N2
Nitrogen. Soaks up heat in the air, and lowers the temperature of a fire. By association, it can very quickly lower the temperature of a fiery rupture to the point where the flames self-extinguish. Proof of this can be seen if you go down to the incinerator with a can of burnmix, and a can of 20% burnmix and 80% N2. The N2 contaminated fire will not burn nearly as hot or as well. This is why the Toxins guide recommends opening up a can of N2 to the air, it can and will save your life if there's a rupture. Due to the vastly higher heat capacity, N2 is incredibly better at stopping fires: in 100 kPa of N2/O2 80/20 and 100 kPa of O2 100, the N2/O2 is effectively 12200 kPa as opposed to 100 kPa in terms of soaking up heat and it doesn't allow the fire to grow in size as quickly, the combination of which can even lead to non-permanent ignition sources being snuffed out by themselves.
CO2
Carbon Dioxide. An invisible, heavy gas, CO2 is one of the first and fastest gases the scrubbers suck out of the air. It chokes people effectively and quickly, and if you can be bothered to set the alarms up, will result in a invisible room that kills those in it. Takes some setup and can be very, very annoying. The emote for this at low levels is gasps.
N2O
Nitrous Oxide, a.k.a. Sleeping Agent. A white-flecked gas. Makes you laugh at low doses and at higher ones puts you to sleep. Scrubbers don't deal with it too well and portable scrubbers just choke on it. If using this as a sleep gas mix do not forget the O2 at at least 16 kPa, or you will kill someone.
Plasma
Toxins. The one truly flammable gas on the station, plasma is purple, and highly toxic. Of note is the fact that in the presence of any oxygen at high pressures, Plasma pumped into air, and Burn Mix (O2 and Plasma), can and will spontaneously ignite in an open area at high pressures.
Physical Characteristics of Gases
Ideal gas law: PV = nRT
Where R (ideal, or universal, gas constant) = 8, the following are linked by this equation. Sadly, without either Volume or Moles, it's not useful in game and is here for the theory.
Pressure (P): Measured in kPa, kiloPascals, Pressure is lethal above 750 kPa's. A pressure in a room above 1000 kPa's necessitates internals to breathe properly.
Volume (V): Another unseen variable, Volume is how much the area/canister/tank or piped tank has space inside it. This helps dictate how much gas it can hold. Volume is essentially the 'mole divider' when converting between a canister/air pump to your tank; having a higher volume essentially makes the tank that much more efficient, proportionally, so an EEOT has twice the contained air per kPa in comparison to a regular EOT.
- Emergency O2 tanks have volume 3.
- Extended Emergency O2 tanks have volume 6.
- The big, blue, tanks have volume 10.
Moles (n): While not a variable that can be seen, Moles are the amount of particles of a gas in the air. It is moles that cause odd effects with a certain chemical. As it dumps so many moles to a tile, to keep the pressure acceptable, the moles have to be very, very cold, causing the infectious effect.
Temperature (T): Measures in K, Kelvin, Temperature above 360 K and below 260 K causes burn damage. Bomb making usually relies on a temperature at or in excess of 90 000 K.
Heat Capacity: A gasmix has heat capacity, and it is calculated by taking into account the quantity of all of the gases in the air and their specific heat. Oxygen has a specific heat of around 20, CO2 has 30, and N2 has 300. When you factor in the normal 70% N2 it leaves you with a very high specific heat. The higher the specific heat, the more energy required to heat up the mixture, meaning that with an air mix vs. pure O2 mix, it takes much more energy to heat the air than the O2, and the increase in energy required also decreases how much the fire spreads. Simply slowing it down means that heat energy will be 'soaked up' by the air instead of super-heating everything extremely quickly.
- Air: 250
- O2: 20
- N2: 300
- CO2: 30
- N2O: 40
- Plasma: 200
Fire: An effect caused by burning plasma, fire comes in two different forms of hotspot. It causes massive burn damage, and a strong fire will not be stopped by standard firesuits. Plumbing N2 into a room might work, but heavy firefighting is not the point of this section. Fire will ignite any form of combustibles in near tiles. Sufficiently hot fires use less oxygen as they rise in temperature. This is due to the fact that fires remove X plasma and X*(1.4-Y, Y< or = 1) oxygen. X CO2 is produced. Ideal Burnmix is: higher O2 than plasma for really cold ones, such as open air small plasma usage, with a consistent decrease based off of temperature until you reach 28.57% O2. In general, 30% O2 is a good mark for N2 less fires.
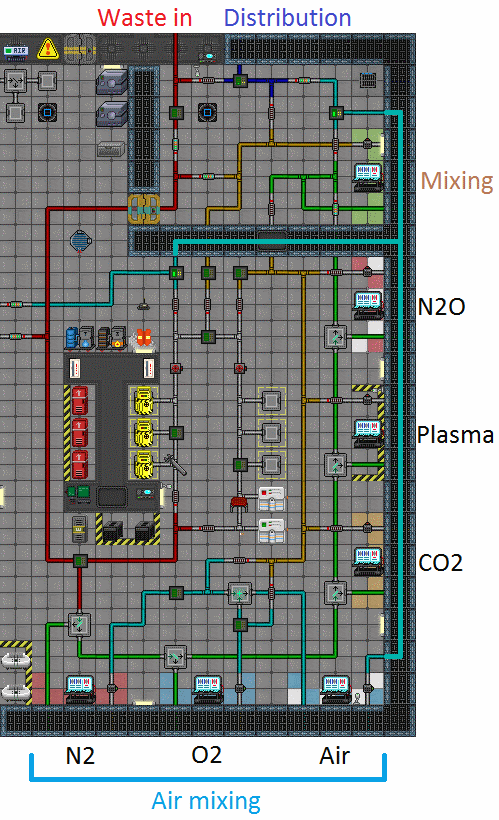
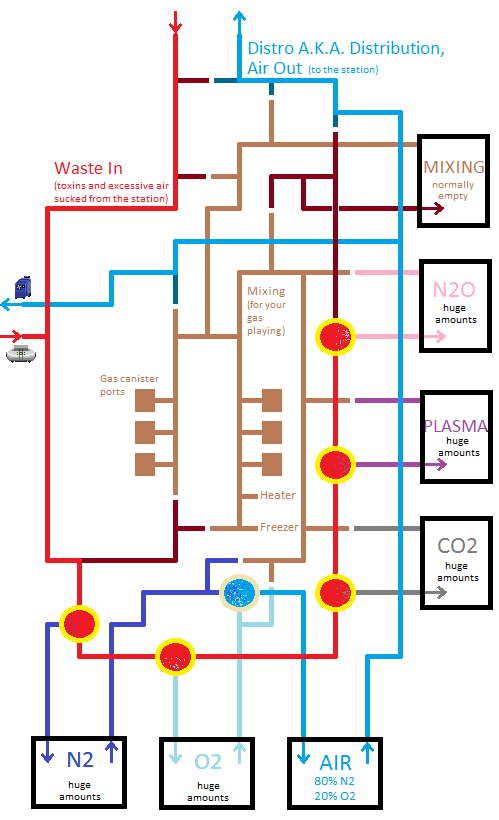
Setting Up Atmospherics
The pictures above show how Atmos starts up, but you don't want it to be like that for too long.
Let's make up an example situation to see how these pipes work:
- Scientist Bill messes up and fills the Toxins Lab with plasma but fortunately manages to evacuate the room safely.
- Being an otherwise ideal situation Atmos-wise, the Toxins Lab's air scrubbers have been set to filter out all hazardous gases (they're not set by default, this has to be done manually or by asking the AI to do it) and plasma starts to get sucked through the scrubber into the waste pipes.
- The plasma finally arrives to the Waste In -loop (the red pipe loop) at Atmos. It travels south through the pipes, its first stop being the N2 Filter.
- If there was any Nitrogen in the waste gas, it would get filtered out here, and the rest of the gas continues its journey through the waste loop, same thing happening at every filter.
- The plasma finally reaches the Plasma Filter.
- Here the plasma gets extracted from the waste gas and pushed into the big plasma tank-room outside the windows.
- The plasma stays in the room until someone decides to pump it out.
- Scientist Bill by now notices that the Toxins Lab has no plasma anymore and is able to continue his work.
MAKING ATMOS AS EFFICIENT AS POSSIBLE:
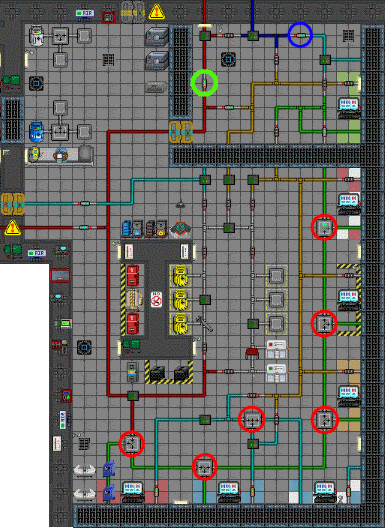
It's about time we stop with the theory and get down to business. The two machines at the top can dispense infinite pipes, and your wrench can disconnect and connect pipes. Remember, you cannot disconnect pumps when they are on.
Next up is a very simple step by step guide how to set up the Atmospherics pipe system to be (nearly) as efficient as possible. Note that this is only one style how to set up the pipes. There are many ways and they have their own pros and cons.
- Get at least two Volume Pumps from the Pipe Dispenser at the north side of Atmos.
- Replace the green circled normal pumps with volume pumps, making the waste gas -system 100x more efficient. We want the waste gas sucked out as soon as possible.
- Replace the blue circled normal pumps with volume pumps as well, but notice; there are risks involved and all of them are covered at the pros and cons -section below.
- Set all red circled filters to maximum pressure (4500 kPa) so waste gas will actually be moved.
- Go through the N2, O2 and Air -computers beside the south wall and set their output to maximum (5066.25 kPa).
- Pros and cons of this setup:
- + In case of a toxin leak, waste gas will be sucked out quickly (if the area's air alarms are set to filter out all the toxins, by default they are NOT).
- + In case of a breach, air will be poured out with a nice pace, helping you re-pressurize the room quicker after the breach is fixed.
- + With this setup, the grifflords cannot fuck up pipes in the maintenance tunnels. In a room with the default 101.3 kPa atmospheric pressure, pipes with more than 303.9 kPa pressure cannot be unwrenched.
- - In case of a breach, until the hole is fixed, you'll probably spend a small while fighting against the huge air current ("space wind") if you don't switch the vents off.
- - If you'd suddenly get a need to modify any pipes (quite unlikely), you'd need to lower the pressure to under 303.9 kPa, which could take a long time.
- - The station is more vulnerable for sabotage through air alarms. Someone can quite easily hack an air alarm somewhere and set the vents to push out air at maximum pressure, resulting in a shitty situation, because sucking out excessive air is slow.
- + In case of a toxin leak, waste gas will be sucked out quickly (if the area's air alarms are set to filter out all the toxins, by default they are NOT).
After All is Done
There is a short list of things which fall under your stead:
- First and by far most important: make sure pipes don't get broken and if they do, fix them.
- Go around swiping your ID on air alarms, setting Plasma and N2O to siphon, and then re-swiping to lock it. You can ask the AI to do this as well, and probably should.
- Fill all the air pumps with air using a volume pump (more air pumps can be found from the locker room).
- Make extreme extended oxygen tanks for internals use (instructions below).
- Least importantly, maintain the disposals system. You can generate pipes, but it needs welding and is generally a pain in the ass. You can also make fun slides, though.
Using an Air Alarm
Air alarms are the central tool of an Atmos Tech outside setting Atmospherics up. To use an air alarm, simply swipe your ID across it.
Panic Syphons: They turn all vents off and set all scrubbers to syphon. You *can* do better than that, but most of the methods involve setting vents to suck, and if you need a panic syphon, then sucking contaminated air into Distro kinda sucks.
Vents: you control vents through the air alarm. There are the following settings:
- External on, Internal off: will drain/add air from the tile the vent is on to make it the correct amount. All air being moved goes into/comes out of the pipe the vent is attached to. Set to 0 to drain air, or pressurise to specific levels.
- Both on: completely useless. Don't bother.
- External off, Internal on: Drains/adds air to the tile to get the pipe attached to the correct level. Setting a vent to internal and the desired pressure to 0 causes ALL gas which enters the pipe to be shunted out onto the tile.
Scrubbers: two settings, scrubbing and syphoning.
- Scrubbers will slowly drain any gases set to scrub in the air of the tile they are on, and transfer it to their pipe. Really, really slow with N2O.
- Syphons will do the same, except indiscriminately and drain all gases on their tile.
Optimizing Internals
- On a basic view, a 16 kPa minimum O2 requirement in internals. Pure O2 is theoretically toxic in real life, but has no representation for this in code, and takes a while to be really dangerous anyway (they use it to treat certain diseases, for example), and thus using an air tank for internals is fairly inefficient.
- Cold air has more moles per kPa, and because people breath in moles, and filling tanks usefully for internals is largely capped by the 1000 kPa release pressure, which means cooling your air before using in internals is important. Cooled down air, such as from a freezer-ed canister, is the most efficient way to set up internals. Cooling it below 264 K will result in icicles inside lungs!
- If you need to empty an internal tank to make space for better, colder air, you can use an air pump. Set it to 'pump in' and 'turn on' then 'off' with the tank inside it, allowing you to refill the tank more effectively.
Fun Projects
- The Atmospherics system is far from optimal, and I'm talking about just the pipe configuration! Break out that wrench and start experimenting (just make sure you know what's what)!
- Extremely high temperature gases (like those from a panic siphoned fire) can really clog the waste loop. Could you do something to correct that?
- No one uses the ports outside of the 'refilling' station, but that doesn't mean that functionality can't be added onto them!
- The wall section that looks like the letter 'I' can be dismantled if you need more working space for pipes.
- Don't count out the grated window areas, they can be a great (har har) way to utilize the vacuum of space without an EVA suit.
- Speaking of EVA suits, your engineering buddies can potentially help you with anything you might want to do in space, be it adding or modifying pipes. Watch the hilarity as that incompetent engineer fumbles with the huge crate of pipes he dragged out into space for you!
- The main cargo area inside Cargo has a laughably small number of vents, and how many times have those dumb dumbs sent the shuttle off while the doors are open?
- The brigs distribution system is set up to be potentially independent of the rest of the stations distribution loop, maybe other places can be set up like this as well?
- The mining station doesn't have air recycling. Very long rounds might make this a problem for any miners working there.
Useful Atmos Trivia
- Using H/E pipes in space you can cool things down to a very low temperature very quickly. By making a cross with two off them you can have two on one tile, which is known as 'sequesteral' cooling.
- Air Filters on currently burning mixes can siphon out heated but PURE O2 and Plasma. Do the O2 first then the plasma, as there is less O2 in a fire and thus it functions faster. This (and H/E) allow you to reach really obscene temperatures.
- Air Filters and H/E allow you to expose gases to the heat of fires (or their CO2 product) but keep/make them pure, allowing for hot N2O or similar.
- Using a small starter flame/heater you can have in pipe combustion.
- Canister bombs are heated Plasma in a canister, with a O2 tank placed in the canister, and then open the valve between them. You will also need to run very, very fast.
- The gas diverted by an air filter has no maximum pressure, and can therefore reach an insane amount. For example, you can filter out the oxygen into one sealed pipe and it will keep rising.
- Pipes at around 300 kPa pressure can be unwrenched, however, devices such as pumps and filters don't really 'hold' pressure and can be unwrenched at any time (assuming they're off)!
- Gas pumps are for precise pressure control, volumetric pumps are for really fast pumping, and passive gates are for having 'one way' manual valves.
The Less Well Know Hazards of Gases
- Any gas at pressure over 1000 kPa will cause you to start suffocating as in a vacuum. You can just use internals, though.
- N2O is invisible at low pressures. If you start giggling, put on your internals to avoid passing out.
- Any gas can displace O2, and less than 16 (also useful for optimizing internals) kPa of oxygen starts the Oxyloss. CO2 can be removed with the scrubbers, but to get rid of N2 simply apply some way of removing gas from the air and adding O2. My personal favorite is 2 air pumps, 3 connectors and a Air Filter and a canister: 1 pump draws in, goes through the connection and filters N2 into the canister, and the rest to the other pump, which expels it. Can also be used for N2O which is only sluggishly scrubbed otherwise.
- Pressure's above 750 kPa do 10 DPS + 5 DPS for every extra 375 kPa above that mark, rounded off. Space suits completely block it all, but there is no other defence.
Being a Traitorous Scum
If you are just trying to flood a gas like plasma, you can essentially do the basic set up with plasma instead of a N2/O2 mix.
- You can hack an air alarm to use it as a non-Atmostech.
- You can C4 the digital valves to let you remove them and shut down AI control, or save a C4 and disable the cameras if you know there's no Cyborgs on the station.
- Using a gas filter turned on to pour large, ever increasing, amounts of gas onto a single connector port has no visible effects, but if you wrench a canister onto it then the canister will almost immediate fill up with the massive pressure buildup, letting you get super-high pressure plasma/CO2/etc canisters to hit area's with.